Which Best Explains Why Water Dissolves Most Salts
So NaCl is the most popular among laymen but you also have some funky ones like LiF K2O. Water consists of two hydrogen atoms and one oxygen molecule connected by a covalent bond to form a charged H20 molecule.

Exothermic Vs Endothermic Chemistry S Give And Take Discovery Express
Stirring increases the rate of dissolution because it brings fresh solvent into contact with the solute Which combination will produce a precipitate.
. The polarity of water molecules enables water to dissolve many ionically bonded substances. O Water is polar and salts form ions in solution O Water is nonpolar and salts form ions in solution O Water has the same density as salts O Water has a different density than salts. The amount of a substance that.
First and foremost lets define our terms. Question 7 180 seconds Q. Thus water best dissolves polar substances including ionic and polar covalent substances.
The positive part of the water molecule the hydrogen part is attracted to the negative part of the salt the chlorine part. The waters partial charges attract different parts of the compound making them soluble in water. Thus sugar and salt gets easily dissolved in water.
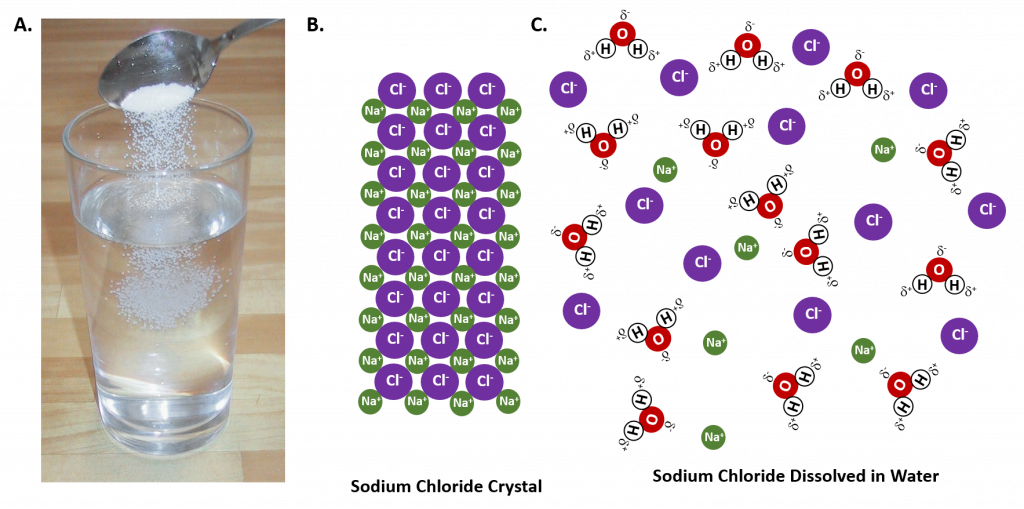
When a salt such as sodium chloride table salt dissolves in water its ionic lattice is pulled apart so that the individual sodium and chloride ions go into solution. Shaking equalizes the water temperature. 1 See answer Advertisement.
Water can dissolve polar covalent compounds through dipole-dipole interactions. To understand this process at the molecular level we must apply the three steps we previously discussed. Salt sodium chloride is made from positive sodium ions bonded to negative chloride ions.
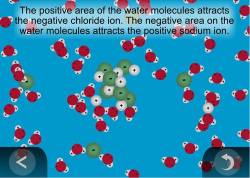
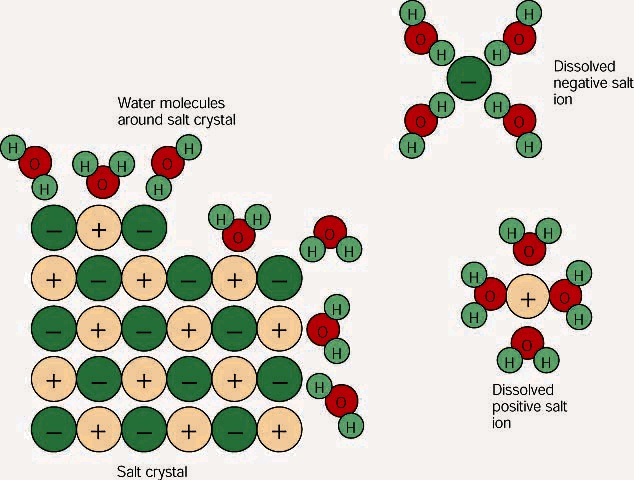
Answer choices Shaking exposes the salts to the solvent more quickly. After the salt compounds are pulled apart the sodium and chloride atoms are surrounded by water molecules as this diagram shows. Because water is a polar molecule each of its ends holds a slight positive or negative electrical charge.
Answer 1 of 35. Which is a cause of polarity in water molecules. These ends attract the positive and negative ions in salt and pull them apart from each other.
Which of the following best explains why shaking the bottle will affect the dissolving rate of the salt. Which of the following best explains why shaking the bottle will affect the dissolving rate of the salt. Salt sodium chloride is made from positive sodium ions bonded to negative chloride ions.
- 25916372 antariusjones8519 antariusjones8519 1 week ago Chemistry High School answered Which best explains why water dissolves most salts. Insoluble salts are ionic compounds that are insoluble in water. Shaking causes more ions to precipitate out of solution.
Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions. This is an interesting question with multiple true answers. The general rule for solvation is like dissolves like.
The salt continues to exist as a solid rather than dissolving in the liquid. A student pours mineral salts into a bottle of cold water. Water is a very polar molecule.
Which statement best explains waters ability to dissolve covalent compounds. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions. It has the most volume.
The biggest similarity between these two substances is that their molecules are charged making them reactive. An ionic solid when dissolved in water then it breaks or dissociates into its constituent particles in the form of ions. This is why salt dissolves in water.
Which best explains why water dissolves most salts. Water can become so heavily attracted to a different molecule like salt NaCl that it can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and thus dissolve it. Water dissolves salt because the positive part of water molecules attracts the negative chloride ions and the negative part of water molecules attracts the positive sodium ions.
Salt to a chemist is an ionic compound. When the sugar or salt are added to water the positive ions anions attracts negative ions of sugarsalt while the negative ions cations attracts postive ions of sugar or salt. Salt as well as any other soluble substance dissolves quicker in hot water because heat makes the water molecules move faster creating more space between them.
Which of the following best explains why sample A dissolves more slowly than the other two. Shaking helps more water evaporate. Hence the molecules of sugar or salt can form a mixture with molecules of water and this is called solubility.
The positively-charged side of the water molecules are attracted to the negatively-charged chloride ions and the negatively-charged side of the water molecules are attracted to the positively-charged sodium ions. If the amount of dissolved solute in a solution at a given temperature is greater than the amount that can permanently remain in solution at that temperature the solution is said to be. Which best describes why water dissolves solid ionic compounds.
Water dissolves salt because the negative part of a water molecule the oxygen part is attracted to the positive part of the salt the sodium part. Which best explains why water dissolves most salts. When salt is mixed with water the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
This extra space means the salt molecules more readily make contact with the water molecules binding to them and creating a solution. Sodium chloride NaCl dissolves when water molecules continuously attack the NaCl crystal pulling away the individual sodium Na and chloride Cl ions. High electronegativity difference between oxygen and hydrogen.
This nonstop attack continuous until the whole NaCl crystal disintegrates. Which best explains why water dissolves most salts. It has the smallest surface area.
By Staff Writer Last Updated April 17 2020 Water dissolves salt by dissociating the ions in salt from each other.

Dissolving And Back Again American Chemical Society
The Facts About River Basin Salinity Irrigation And Salinity Rivers Water Quality Land Quality Evaporation Ponds Victor Miguel Ponce

Why Does Water Dissolve Salt Youtube

Water Molecules And Their Interaction With Salt U S Geological Survey

Dissolving Process Chemistry For Non Majors

When Salts Are Dissolved In Water They Lisbdnet Com
Dissolving And Back Again American Chemical Society

Water Dissolves All Nitrate Salts And Most Chloride Salts True Or False
What Happens When You Stir Salt Into Water Asset
Ch150 Chapter 7 Solutions Chemistry
Do Materials Dissolve Faster In Salt Water Or Regular Water Quora
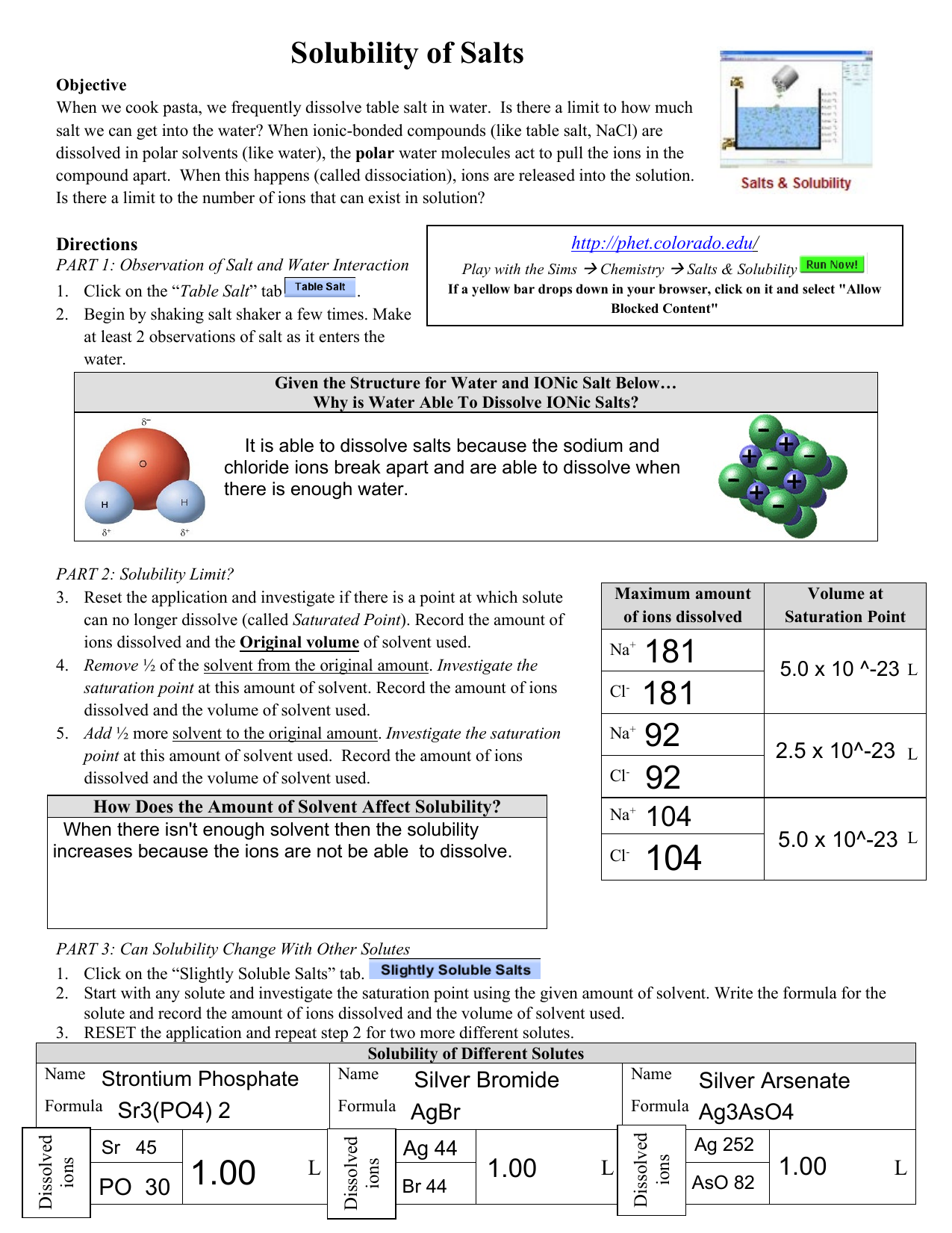
Chem Worksheet Solubility And Salts

Water 5 Grade 5 Curriculum The Inquiry Project

Can Liquids Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
Why Is The Dissolving Of Anhydrous Salts And Acids In Water An Exothermic Physical Process I Just Don T Get It Quora

When Sugar Or Salt Is Dissolved In Water What Will Be Change In Their Volume Why Quora

Comments
Post a Comment